Medical robotics is changing rapidly. With the convergence of three new emerging technologies, procedures that were never possible are feasible.
The IGAR system is validated in the MRI environment (1.5 and 3.0 T)
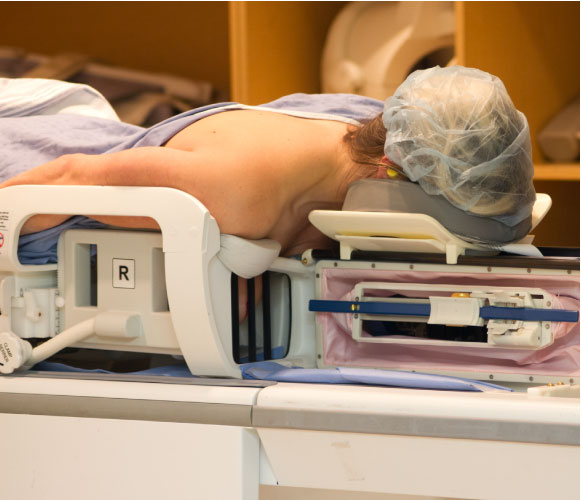
Our robot is FDA 510(k) cleared for use in MRI-guided breast biopsy and has been demonstrated in 30 patients. The IGAR technology is supported with a strong supporting IP estate of filed applications and issued patents in the United States, Canada, and the European Union.
With new clinical scenarios and more flexible clinical access
Thanks to the rise of smaller, more portable MRI machines, the costs and wait times for clinical access are decreasing.
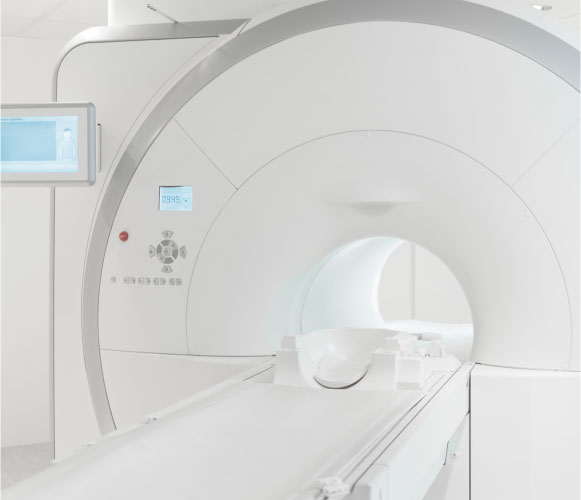

IGAR's compact, portable design fits inside standard MRI bores.
Our robotics system automatically executes the clinical plan with more accurate and precise control, increasing physician autonomy while standardizing skill requirements and reducing burden on staff.
The IGAR technology platform may be adapted for future products that could move seamlessly with you into standard OR environments, giving you the right image guidance at the right time for procedures.
This functionality would help to optimize the use of valuable clinical resources.
With new clinical scenarios and more flexible clinical access
Thanks to the rise of smaller, more portable MRI machines, the costs and wait times for clinical access are decreasing.

Our MRI-compatible robot, IGAR, has clinically demonstrated its use in breast bioposies for breast cancer patients. Other possible applications include targeted therapeutic delivery and device placement

IGAR moves seamlessly with you into standard OR environments, giving you the right image guidance at the right time for procedures. This functionality helps to optimize the use of valuable clinical resources.

We're designing and building robotics that work where care is most needed: In an MRI bore, in standard OR environments and—one day—even office settings.

