Read the published studies on the only FDA-cleared* robot for use with MRI up to 3T.
* FDA cleared for breast biopsy. Glenn is under development and is not FDA cleared for prostate indication in the US or available for sale.
When it comes to soft tissue cancers, there’s no substitute for the image quality and clarity of MRI. Yet, MRI becoming an interventional visualization tool—more than just a diagnostic—for advancing precision in cancer care was unimaginable.
Meet GlennTM
We envision a future of cancer care that imposes less on patients and minimizes side effects with the assurance of finding and treating the cancer. So, patients won’t have to choose between peace of mind in knowing the cancer is treated and doing the things that matter most to them.
For prostate cancer, that means striving to work within the confines of the prostate capsule—as small as a walnut—in order to treat the cancer.
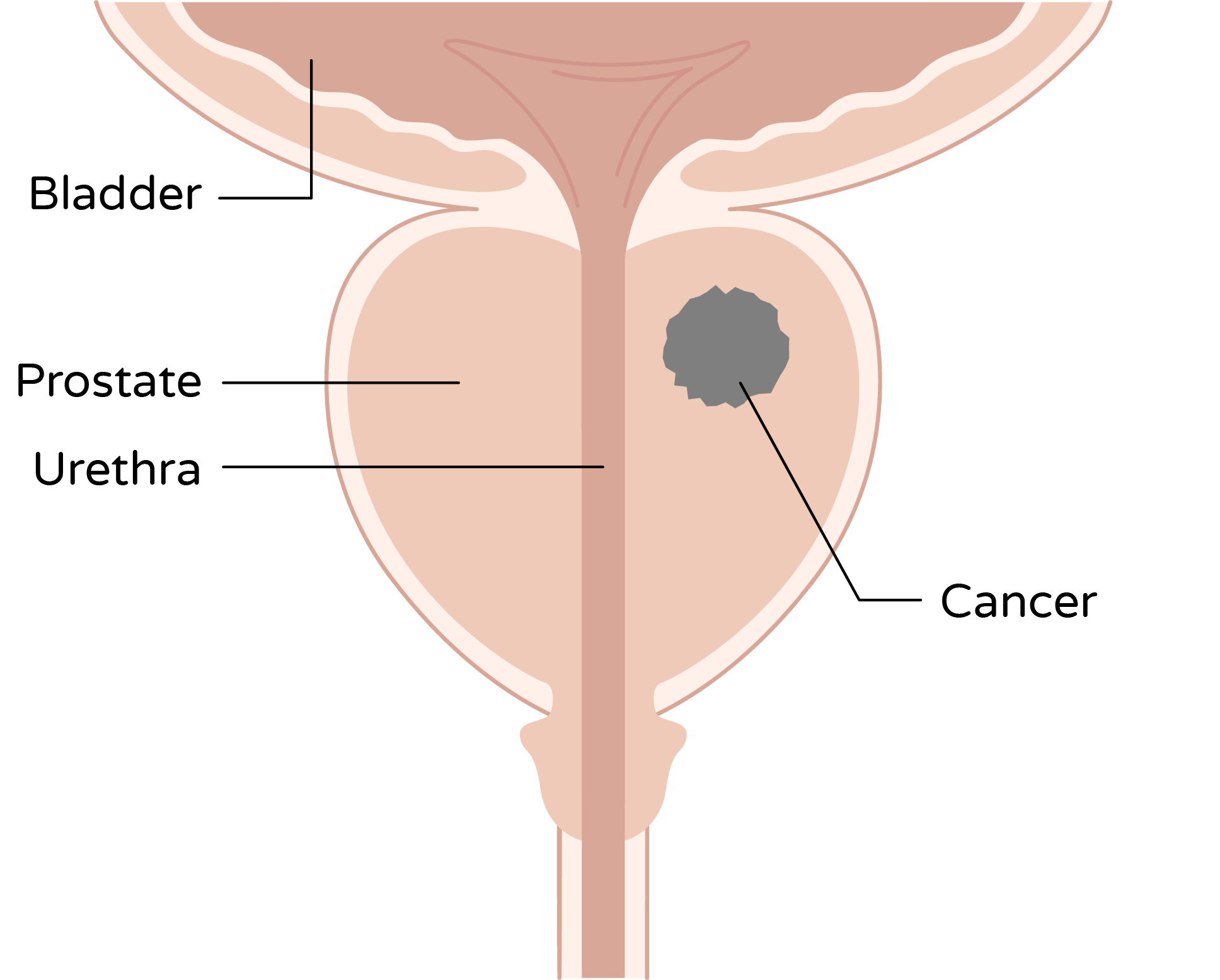
Urologists seeking better precision for their patients and tired of MRI substitutes know there’s nothing like the real thing when it comes to seeing clearly.
But, interventional MRI has been impractical and inaccessible for urologists.
Until now...
