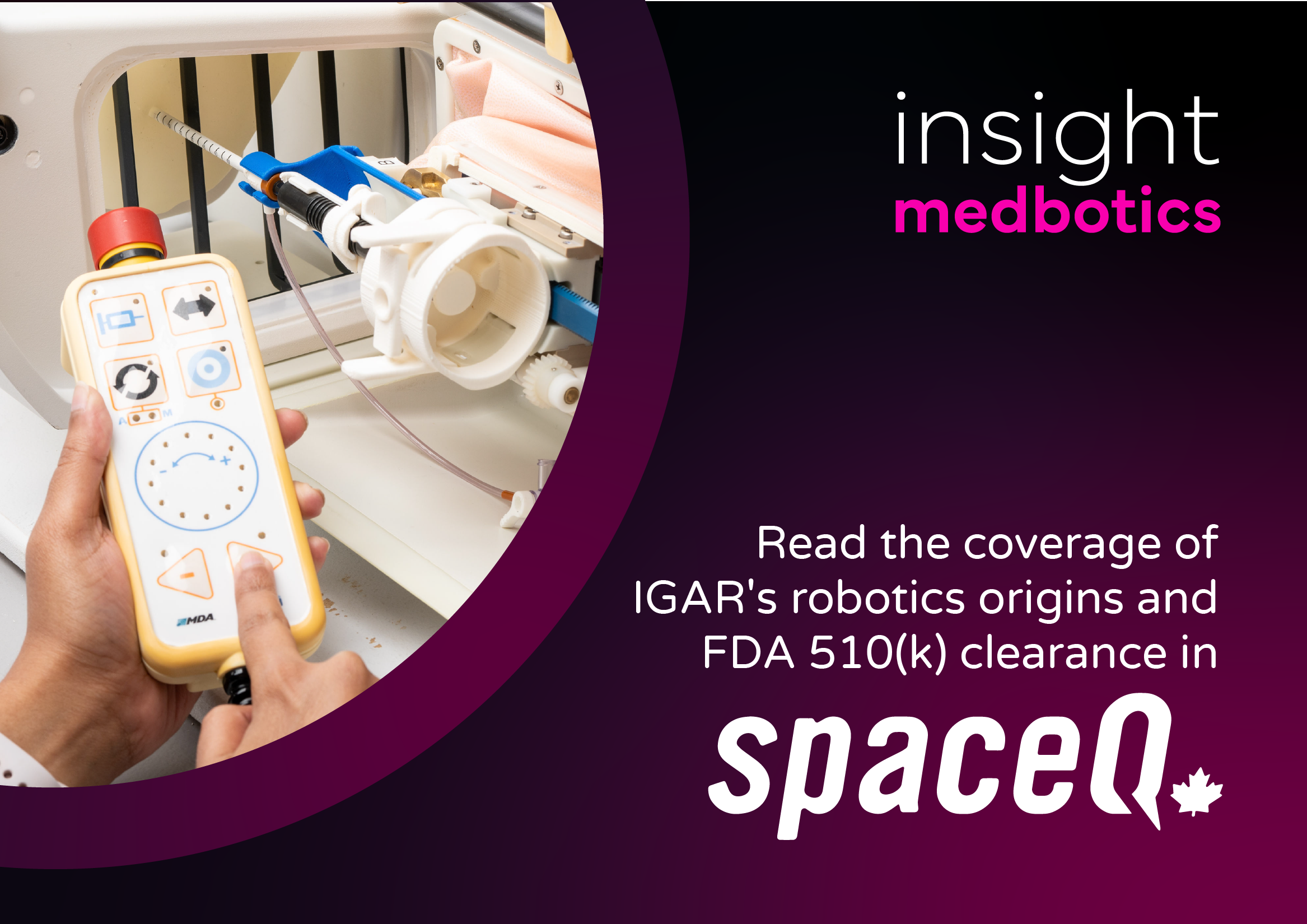
Thanks to SpaceQ for this comprehensive IGAR’s technical development from the benchside to a successful FDA 510(k) clearance in breast biopsy.
Craig Bamford’s article outlines the partnership between Insight Medbotics, MDA and the Centre for Surgical Invention and Innovation (CSii) to drive this achievement, and the leadership of Dr. Mehran Anvari along the way.
We do wish to note that IGAR is not currently for sale in the U.S. market.
Read the full piece, “Canadarm-based medical robot passes FDA review,” on the SpaceQ website.